What is Tremfya (Psoriatic Arthritis) Tremfya?

Tremfya is a medication used to treat psoriatic arthritis, which is a chronic inflammatory disease that affects the joints and skin. This medication is specifically designed to target the underlying cause of psoriatic arthritis, which is an overactive immune system.
Tremfya belongs to a class of drugs called biologics, which are made from living cells and work by blocking specific proteins that contribute to the inflammation process. This medication is administered by injection and is typically given once every 8 weeks.
Clinical studies have shown that Tremfya can significantly improve the symptoms of psoriatic arthritis, including joint pain, stiffness, and swelling. It has also been shown to improve skin symptoms associated with the disease.
While Tremfya is generally considered safe and effective, it may not be suitable for everyone. It is important to discuss any pre-existing medical conditions or medications with your healthcare provider before starting treatment with Tremfya. Additionally, patients taking Tremfya may experience side effects, including injection site reactions, infections, and allergic reactions.
In conclusion, Tremfya is a medication that has been shown to be effective in treating psoriatic arthritis. It works by blocking specific proteins that contribute to inflammation, thereby reducing joint pain, stiffness, and skin symptoms associated with the disease. However, it is important to discuss the risks and benefits of this medication with your healthcare provider before starting treatment.
Frequently Asked Questions about tremfya (psoriatic arthritis) tremfya
Tremfya 100 mg solution for injection in pre-filled pen Each pre-filled pen contains 100 mg of guselkumab in 1 mL solution. Guselkumab is a fully human immunoglobulin G1 lamda (IgG1λ) monoclonal antibody (mAb) produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology.
adjective. tri·um·phant trī-ˈəm(p)-fənt. 1. : victorious, conquering.
TREMFYA® is a prescription medicine used to treat adults with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet or UV light). TREMFYA® is a prescription medicine used to treat adults with active psoriatic arthritis.
Generic Name: guselkumab
This medication is used to treat plaque psoriasis and psoriatic arthritis. Guselkumab belongs to a class of drugs known as monoclonal antibodies.
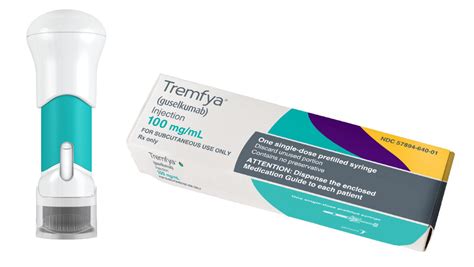
Tremfya belongs to a class of medications called interleukin-23 blockers. A class of drugs is a group of medications that work in a similar way. Tremfya comes as a liquid solution inside single-dose prefilled syringes and single-dose One-Press injectors.
TREMFYA® is administered as a 100 mg subcutaneous injection once every 8 weeks, after starter doses at weeks 0 and 4. In active psoriatic arthritis, TREMFYA® may be administered alone or in combination with a cDMARD (e.g., methotrexate). TREMFYA® is intended for use under the guidance and supervision of a physician.
Mechanism of Action
Tremfya is a human monoclonal IgG1λ antibody that selectively binds to the p19 subunit of interleukin 23 (IL-23) and inhibits its interaction with the IL-23 receptor. IL-23 is a naturally occurring cytokine that is involved in normal inflammatory and immune responses.
These are just a few of the more common side effects reported by people who took Tremfya in clinical trials:
- upper respiratory infection.
- headache*
- injection site reactions*
- joint pain.
- diarrhea.
TREMFYA® TREATMENT EVERY 8 WEEKS, AFTER 2 STARTER DOSES:
TREMFYA® is given as a 100 mg injection under your skin that you only need to take every 8 weeks, after 2 starter doses at weeks 0 and 4.
The best place to inject Tremfya is into the front of your thighs or the lower part of your abdomen, avoiding the area around your belly button (stay an inch away from your belly button). If somebody else is giving you your injection they can also administer it into the outer area of your upper arm.
Tremfya contains the active ingredient guselkumab, and it's available only as a brand-name biologic drug. It doesn't come in a biosimilar version. A biosimilar medication is a drug that's similar to a brand-name biologic drug (the parent drug). Also, biosimilars tend to cost less than brand-name medications.
Tremfya (guselkumab) is a member of the interleukin inhibitors drug class and is commonly used for Plaque Psoriasis, Psoriasis, and Psoriatic Arthritis. The cost for Tremfya subcutaneous solution (100 mg/mL) is around $13,922 for a supply of 1 milliliter(s), depending on the pharmacy you visit.
Generic Name: guselkumab
This medication is used to treat plaque psoriasis and psoriatic arthritis. Guselkumab belongs to a class of drugs known as monoclonal antibodies. It works by blocking a certain natural substance in your body (interleukin-23) that may cause inflammation and swelling.
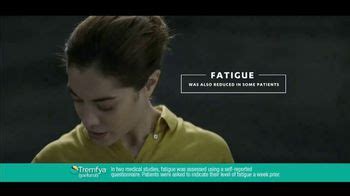
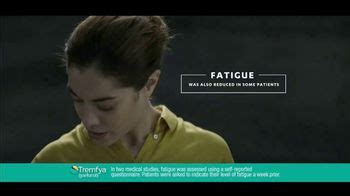
In two medical studies, more than 5 out of 10 people who took TREMFYA® had at least a 20% improvement in active PsA symptoms (joint pain, stiffness, swelling) at 24 weeks compared to placebo. Individual results may vary. One medical study showed that less joint pain, stiffness, and swelling is possible even at 2 years.
Tremfya can still increase a person's risk of infection or cause reactivation of tuberculosis; however, the risk for serious infection is low (0.3%) and similar to a placebo (a medicine with no active ingredient in it).
TREMFYA® is a single-dose 100 mg injection taken under the skin at weeks 0 and 4, and then every 8 weeks. That's only 6 doses per year after 2 starter doses. TREMFYA® is intended for use under the guidance and supervision of physicians. Patients may self-inject after proper training and physician approval.