What the Tremfya TV commercial - Stiff Joints is about.
The Tremfya TV Spot, 'Stiff Joints' is an advertisement that focuses on the treatment of psoriatic arthritis. The commercial highlights the debilitating effects that the condition can have on patients, such as stiff joints, difficulty moving, and pain. The ad goes on to explain how the medication, Tremfya, can alleviate these symptoms and help patients manage their condition more effectively.
The opening scene shows a woman struggling to move and her joints stiffening up as she reaches for a glass of water. She is shown alone in her home, highlighting the isolation that patients with psoriatic arthritis may feel. The voiceover then explains that psoriatic arthritis is a chronic autoimmune disease that causes painful and stiff joints.
As the ad progresses, the woman is shown receiving treatment with Tremfya and how it has helped her manage her condition. She is now able to move more easily and participate in activities that she once struggled with. The voiceover emphasizes the role that Tremfya plays in helping patients achieve better outcomes for their psoriatic arthritis.
The commercial concludes with a message encouraging patients to speak to their doctor about Tremfya and how it may be able to help them manage their psoriatic arthritis symptoms. Overall, the Tremfya TV Spot, 'Stiff Joints' is an informative and heart-warming advertisement that sheds light on the debilitating nature of psoriatic arthritis and the benefits of Tremfya in treating the condition.
Tremfya TV commercial - Stiff Joints produced for
Tremfya (Psoriatic Arthritis)
was first shown on television on April 1, 2022.
Frequently Asked Questions about tremfya tv spot, 'stiff joints'
One medical study showed that less joint pain, stiffness, and swelling is possible even at 2 years. For patients who continued on TREMFYA® for 2 years, a similar percentage (7 out of 10 patients) saw a 20% improvement in PsA symptoms at 2 years.
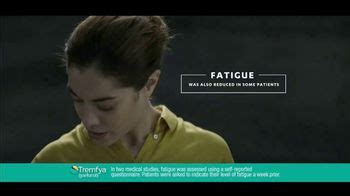
Tremfya isn't known to cause fatigue (lack of energy). However, fatigue could be a sign of an infection or other condition that requires treatment. If you have fatigue while taking Tremfya, tell your doctor. They can look into possible causes.
By blocking the effects of IL-23, Tremfya helps control the release of IL-17 and TNF-α, which reduces inflammation.
This is how the drug helps lessen symptoms of psoriatic arthritis and plaque psoriasis. Tremfya has a half-life of about 15 to 18 days. This means that it takes 15 to 18 days for your body to clear half a dose of Tremfya. A drug is fully cleared from your body after about five half-lives.
Serious side effects of Tremfya
- Serious infection. * Symptoms can include: fever, sweats, or chills. cough. sores on your body that are different from those of psoriasis, which the drug treats. diarrhea. shortness of breath. blood in your phlegm.
- Severe allergic reaction.†
Almost one-quarter (23%) of participants reported a 50% improvement in tender and swollen joint counts (called an ACR50) by week 16 which had increased to 30% by week 24. 8% of participants achieved an ACR70 (70% improvement) by week 16 and 12% achieved an ACR70 by week 24.
Mild side effects of Tremfya
Mild side effects that have been reported with Tremfya include: mild infections,* including upper respiratory infection, fungal skin infection and herpes simplex infection. joint pain. diarrhea.
Psoriatic Arthritis - It can take a number of weeks before a person's psoriatic arthritis symptoms improve on Tremfya. An assessment will be made after 16 weeks of treatment, and then if the psoriatic arthritis has not responded adequately following 24 weeks of treatment, Tremfya will be stopped.
Upper respiratory infection. Tremfya may cause an upper respiratory infection such as the common cold. This is because Tremfya can weaken your immune system and make it less able to fight off germs that cause infection. Upper respiratory infections were the most commonly reported side effect in studies of Tremfya.
Official answer. It takes about 16 weeks for Tremfya to start working in most people, which is two doses of Tremfya because each dose is given 8 weeks apart.
The most common side effects of TREMFYA® include: upper respiratory infections, headache, injection site reactions, joint pain (arthralgia), diarrhea, stomach flu (gastroenteritis), fungal skin infections, herpes simplex infections, and bronchitis.
Serious adverse events occurred in 1.9% of subjects in the TREMFYA group (6.3 events per 100 subject-years of follow-up) compared to 1.4% of subjects in the placebo group (4.7 events per 100 subject-years of follow-up), and in 2.6% of subjects in U.S. licensed adalimumab group (9.9 events per 100 subject-years of ...