Afrezza Rapid Acting Insulin tv commercials
TV spots
TV commercials Afrezza Rapid Acting Insulin

Advertisers
Advertisers of commercials featuring Afrezza Rapid Acting Insulin
Afrezza
What is Afrezza?Afrezza is a rapid-acting inhaled insulin that is designed for people with diabetes who require insulin to manage their blood sugar levels. It is the only inhaled insulin available on...
Actors
Actors who starred in Afrezza Rapid Acting Insulin commercials
What is Afrezza Rapid Acting Insulin?
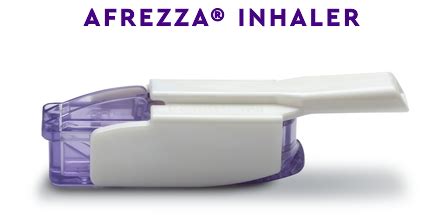
Afrezza is a brand of ultra-rapid acting inhaled insulin used for glucose management in adults with type 1 or type 2 diabetes. It was approved by the US Food and Drug Administration (FDA) in 2014 and is the only fast-acting inhaled insulin available.
Afrezza delivers insulin through an oral inhaler and single-use cartridges , making it a convenient alternative to traditional insulin injection methods. It is taken at the beginning of a meal and its effects are felt within minutes, allowing for more precise blood sugar management.
This type of insulin is a good option for individuals who have difficulty with traditional insulin injection methods. Afrezza may also be a good option for individuals who need a mealtime insulin but prefer not to take injections.
It's important to note that Afrezza is not suitable for people who have hypersensitivity to regular human insulin. Careful monitoring of blood sugar levels is also necessary when taking Afrezza, as with any other type of insulin.
Overall, Afrezza is a fast and convenient option for individuals with diabetes who require mealtime insulin. Patients can get more information about Afrezza from their healthcare providers or from the Afrezza website.