What is Neulasta Onpro?

Neulasta Onpro is a medication used to help prevent febrile neutropenia in patients undergoing chemotherapy. Febrile neutropenia is a condition where a person experiences a fever and low white blood cell count while undergoing chemotherapy, which can increase the risk of infection and hospitalization. Neulasta Onpro contains pegfilgrastim, which is a synthetic version of a protein made by the body that stimulates the production of white blood cells.
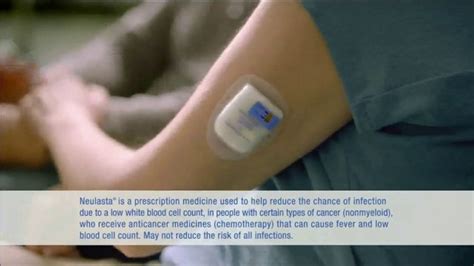
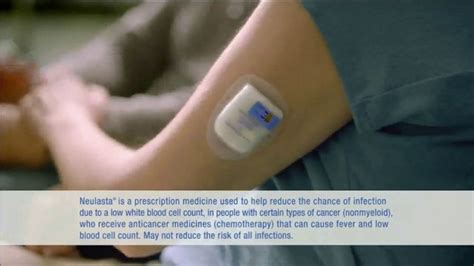
Neulasta Onpro is administered using an on-body injector that is worn on the skin and automatically delivers the medication at a predetermined time. This eliminates the need for patients to return to the doctor's office for additional injections, which can be particularly beneficial for patients who live far from their healthcare provider or have transportation difficulties.
The Neulasta Onpro kit comes with a prefilled syringe containing the medication and the on-body injector device. The prefilled syringe should be kept in the kit carton until used to protect it from light. The on-body injector device should be applied to the skin of the patient's abdomen, upper arm, or thigh. Once applied, the device will deliver the medication over the course of approximately 45 minutes.
Overall, Neulasta Onpro is an important medication for patients undergoing chemotherapy who are at risk for febrile neutropenia and inconvenience of multiple doctor visits for injections. However, as with any medication, it is important to consult with your healthcare provider to see if Neulasta Onpro is appropriate for your specific situation.
Frequently Asked Questions about neulasta onpro
Neulasta is used to help prevent neutropenia in people who're receiving certain cancer treatments. The drug helps to reduce the risk of serious infections during cancer treatment. For example, if you have breast cancer and receive the chemotherapy drug carboplatin, your doctor may prescribe Neulasta.
Neulasta is a man-made form of granulocyte colony-stimulating factor (G-CSF), which is made using the bacteria Escherichia coli. G-CSF is a substance produced by the body. It stimulates the growth of neutrophils (nu-tro-fils), a type of white blood cell important in the body's fight against infection.
Pegfilgrastim is manufactured at Amgen Inc., Thousand Oaks, California, USA.
What Is Neulasta? Neulasta (pegfilgrastim) is a colony-stimulating factor, a man-made form of a protein that stimulates the growth of white blood cells, used to decrease the incidence of infection, by treating neutropenia, a lack of certain white blood cells caused by receiving cancer chemotherapy.
Neulasta® is a prescription medicine used to help reduce the chance of infection due to a low white blood cell count, in people with certain types of cancer (non-myeloid), who receive anti-cancer medicines (chemotherapy) that can cause fever and low white blood cell count.
For Neulasta to have its best effect, it needs to go into the body 24 to 72 hours after chemotherapy.
Amgen is committed to helping clinically appropriate patients access Neulasta.
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each pre-filled syringe contains 6 mg of pegfilgrastim* in 0.6 mL solution for injection. The concentration is 10 mg/mL based on protein only**. * Produced in Escherichia coli cells by recombinant DNA technology followed by conjugation with polyethylene glycol (PEG).
It serves to stimulate the production of white blood cells (neutrophils). Pegfilgrastim was developed by Amgen.
It works by helping your body make more white blood cells, which protects your body from infection.
Neupogen (filgrastim) is a short-acting medication that's given daily for several days. Neulasta (pegfilgrastim) is a longer-acting medication that's administered only once after a chemotherapy session. Neupogen and Neulasta are both available as reference medications.
The reason for this minimum 24-hour delay is to allow the body time to metabolize and excrete the cytotoxic drugs. Otherwise, there is a risk that enough chemotherapy will still be present in circulation to kill many of the neutrophils that are produced as a result of giving G-CSF.
Approval Date: 1/31/2002.
Pegfilgrastim is a covalent conjugate of recombinant methionyl human G-CSF (filgrastim) and monomethoxypolyethylene glycol. Filgrastim is a water-soluble 175 amino acid protein with a molecular weight of approximately 19 kilodaltons (kD).
First developed by Amgen, pegfilgrastim was initially approved by the FDA in 2002 and marketed as Neulasta.
Approval Date: 1/31/2002.